✅ Key Features:
-
Compliance & Standards:
-
Meets FDA, ICH Q1A & cGMP standards
-
Supports long-term, accelerated, and intermediate stability studies
-
Validation documentation – DQ, FAT, IQ, OQ, PQ with calibration certificates
-
-
Construction & Controls:
-
Advanced refrigeration, humidification, dehumidification, and air handling system
-
PID/PLC based multifunctional controller with touch screen interface
-
Auto changeover of standby systems for uninterrupted operation
-
Ethernet networking, PC & printer interface for real-time monitoring
-
-
Standard & Special Conditions:
-
Pre-set stability conditions: 25°C/60%RH, 30°C/65%RH, 40°C/75%RH
-
Optional custom configurations: 25°C/40%RH, 30°C/35%RH, 40°C/15%RH, 55°C/20%RH, 55°C/75%RH
-
-
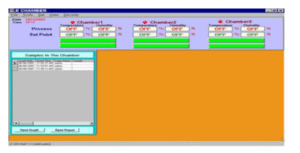
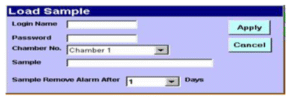
Data Management (21 CFR Part 11 Compliant):
-
Secure electronic records & signatures
-
Password-protected logins & authority-based access control
-
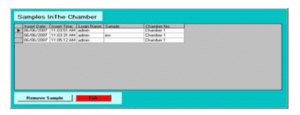
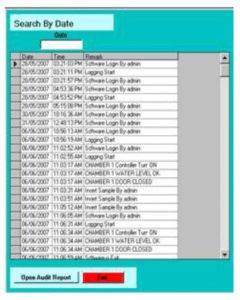
Time-stamped audit trails for batch operations, alarms, configuration changes
-
Export-ready reports with Excel conversion
-
-
Performance & Accuracy:
-
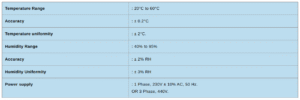
Temperature Range: 20°C to 60°C, ±0.2°C accuracy
-
Temperature Uniformity: ±2°C
-
Humidity Range: 40% to 95%RH, ±2%RH accuracy
-
Humidity Uniformity: ±3%RH
-
-
Safety & Reliability:
-
Standby refrigeration, humidity, and sensor backup
-
Audio-visual alarms for deviations and water level monitoring
-
Overload protection & safety temperature controller
-
SMS alerts for alarms and process values (single/multi-user)
-
📦 Technical Specifications:
-
Data Logger: 8 or 16 channel calibrated logger (temperature & humidity mapping, validation reports)
-
Power Supply: 230V ±10%, 1 Phase AC, 50Hz OR 440V, 3 Phase AC
-
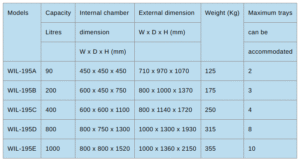
Capacity: Custom-built to laboratory or industrial requirements
-
Validation: DQ/FAT/IQ/OQ/PQ with calibration certificates
🧪 Applications:
-
Pharmaceutical stability testing
-
Biotechnology product incubation
-
Drug and vaccine research
-
Chemical stability and life cycle evaluation
-
Accelerated aging and shelf-life studies











Reviews
There are no reviews yet.